Explain Difference Between Reducing and Nonreducing Sugars With an Example
Those carbohydrates which contain free aldehyde or ketonic group reduce Fehlings solutions and Tollens reagent are called reducing sugar eg monosaccharides like glucose fructose galactose are reducing sugarsThose carbohydrates which do not contain free aldehydes or ketonic group and do not reduce Fehlings solution and Tollens reagent are called non-reducing sugars eg. Moreover the presence of free aldehyde group makes it a reducing sugar.
Difference Between Reducing And Nonreducing Sugar Definition Chemical Properties
Difference Between Reducing Sugar And Non-reducing Sugar In Tabular Form.
. Thus reducing sugars can be detected using the Benedicts test as they reduce the soluble copper sulphate to insoluble brick-red copper oxide. Carbohydrates containing free aldehyde and keto functional group are thus reducing sugars. If it is non reducing the solution will stay blue.
What is another example of non reducing sugar. The characteristic property of nonreducing sugars is that in basic aqueous medium they do not generate any compounds. Nonreducing sugar A sugar that cannot donate electrons to other molecules and therefore cannot act as a reducing agent.
The presence or absence of non-reducing sugars cannot be identified by different tests. Summary The core difference between reducing sugar and non-reducing sugar is that reducing sugars are typically used as reducing agents whereas non. A non-reducing sugar does not have a free aldehyde or ketone and therefore it cannot act as a reducing agent.
Generally the molecular weight of reducing sugars is relatively high when compared to that of reducing sugars. Thus two conditions allow us to identify. A nonreducing sugar is a carbohydrate that is not oxidized by a weak oxidizing agent an oxidizing agent that oxidizes.
Examples are maltose and lactose. The samples which have reducing sugar as its result are glucose and hydrolyzed sugar. Examples of Reducing Sugar.
The main difference between reducing and nonreducing sugar is that reducing sugars have free aldehyde or ketone groups whereas nonreducing sugars do not have free aldehyde or ketone groups. SDS is a denaturant and it coats the protein with charge so that intrinsic charge no longer matters and the proteins migrate according to size. What is a Reducing Sugar Definition Chemical Properties 2.
But SDS does not break disulfide bonds under non-reducing conditions and if your protein has this it could mess up your gel because then 3D shape will matter and the proteins wont just be moving by size. For example maltose 4-O-α-D-glucopyranosyl-D-glucopyranose is a reducing sugar because it has a hemiacetal group. Answer 1 of 7.
Aldehydes and keto groups have reducing character and reduce Tollens reagent and Fehlings Benedicts solution. Non-reducing sugars cannot donate electrons therefore they cannot be. Benedicts test is used for identifying if the given sugar is reducing or non-reducing in nature.
Reducing sugars have the capacity to reduce cupric ions of Benedicts or Fehling solution to. Some disaccharides are reducing sugars they can be oxidised by mild oxidising agents. However under non reducing conditions the interactions are preserved.
Non-reducing sugars do not give a red color but remains green in color when it reacts with Benedicts solution. What is reducing sugar. Reducing sugar form osazones while non-reducing does not form osazones.
The molecular weight of reducing sugars is relatively high when compared to. Sucrose is the most commonly known non-reducing sugar and the chemical structure of sucrose does not allow the formation of the hemiacetal. Examples of non-reducing sugars.
Not all samples have reducing sugars some samples have non-reducing sugar if test on benedict solution. The main difference between reducing sugar and starch is that reducing sugar can be either a mono- or disaccharide which contains a hemiacetal group with a one OH group and one O-R group attached to the same carbon whereas starch is a polysaccharide consisting of numerous glucose units joined by glycosidic bonds. Following are the examples of non-reducing sugar.
Reducing sugars can donate electrons the carbonyl group becomes oxidised the sugars become the reducing agent. Sucrose is the most common nonreducing sugar. Under reducing conditions interactions between two polypeptides is disrupted.
If it is reducing sugar the solution will turn red from blue. A nonreducing disaccharide has no free hemiacetal units. It is mainly monosaccharides while non-reducing is mainly polysaccharides.
A reducing disaccharide contains a free hemiacetal unit. Because the bond involves both anomeric carbons neither carbon has an OH group. Sucrose is an example of a non-reducing sugar.
For starch it only has a small amount of reducing sugar but non-reducing sugar covers the most part of the sample. A nonreducing sugar is a carbohydrate that is not oxidized by a weak oxidizing agent an oxidizing agent that oxidizes aldehydes but not alcohols such as the Tollens reagent in basic aqueous solution. Therefore sucrose cannot reduce other compounds and is not a reducing sugar.
What is a Nonreducing Sugar Definition Chemical Properties 3. Some disaccharides are non-reducing sugars they can NOT be oxidised by mild oxidising agents. Non-reducing sugars have a less sweet taste.
Difference Between Ketose And Aldose. Examples of reducing sugars are fructose glucose and maltose whereas a non-reducing sugar example is sucrose. Glucose- It serves as the main source of energy as it can be directly absorbed in the bloodstream.
The linkage between the glucose and fructose units in sucrose which involves aldehyde and ketone groups is responsible for the inability of sucrose to act as a reducing sugar. Any carbohydrate which is capable of being oxidized and causes the reduction of other substances without having to be hydrolysed first is known as reducing sugar but those which are unable to be oxidised and do not reduce other substances are known as no.
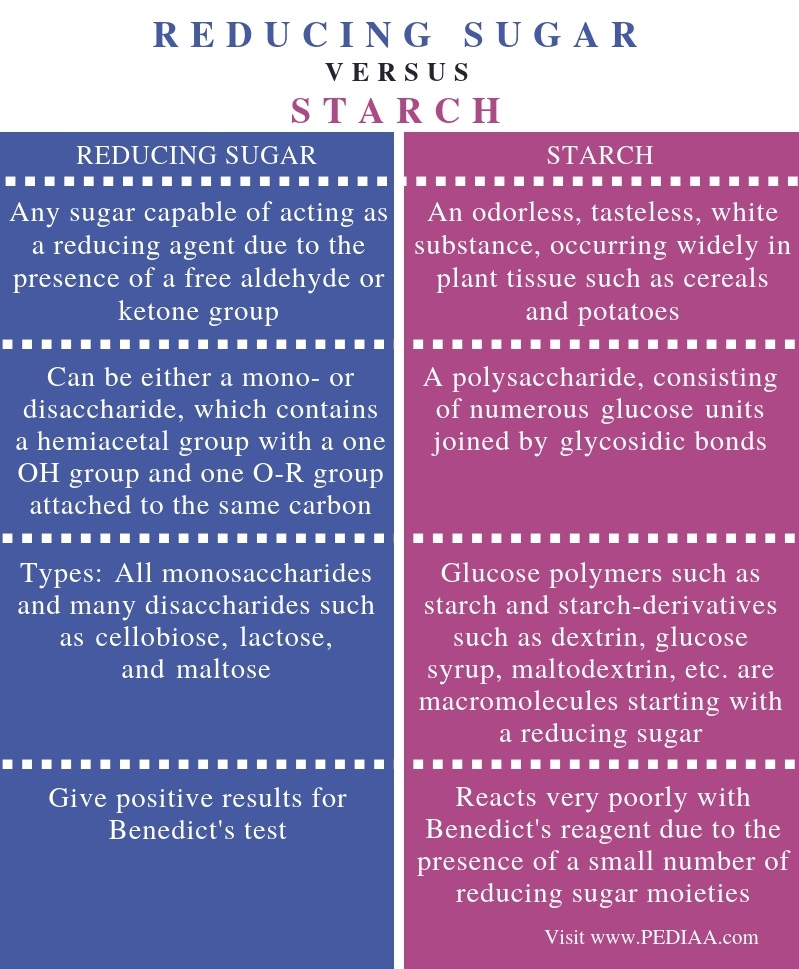
What Is The Difference Between Reducing Sugar And Starch Pediaa Com
Difference Between Reducing And Nonreducing Sugar Definition Chemical Properties

No comments for "Explain Difference Between Reducing and Nonreducing Sugars With an Example"
Post a Comment